 Image source: CDC
Image source: CDC
Commercially available soaps and detergents can destroy the bird flu virus, and possibly the COVID-19 causing novel coronavirus as well.
Previous research in Pakistan has reported that commonly used soap (Lifebuoy) and detergent (Surf Excel) can kill the bird flu virus (H5N1). In this study, published in the 28th March 2009 issue of the Virology Journal, these soaps and detergents were used in different concentrations to determine their ability to kill the bird flu virus. This study found that common soap and detergent brands could destroy the virus at a minimum soap/detergent concentration of 0.1 percent in 5 minutes, and almost immediately at higher concentrations.
“Both bird flu virus and COVID-19 causing novel coronaviruses are enveloped viruses, and belong to the othromyxoviridae and coronaviridae family of viruses, respectively. Therefore, the mechanism by which bird flu virus is killed by soaps and detergents may equally apply to the coronaviruses, including the recent novel coronavirus 2019-nCoV,” microbiologist Dr. Muhammad Akbar Shahid said.
Therefore simple washing, with soaps and detergents, of the poultry shed floors, equipment, transport vehicles, and workers’ clothes can help in containing the bird flu virus. Similarly, washing of hands with soaps and washing of clothes with detergents can also prevent the spread of novel coronavirus (2019-nCoV).
The researchers had claimed that their study was the first published peer-reviewed study on the use of commercially available soaps and detergents to kill the bird flu-causing H5N1 virus; however, this practice was suggested as early as 2005. A similar study on the effect of soaps and detergents to destroy the novel coronavirus (2019-nCoV) can also be performed; however, the results would not be very different from this study on the H5N1 virus.
Along with testing soap brands such as Lifebuoy and detergents such as Surf Excel, other factors including heat, ultraviolet light, and pH were also studied to determine their effect on the bird flu virus. Here is the full article to read.
Disinfectants such as formalin, iodine, and phenol could kill the bird flu virus in 15 minutes at 0.2 to 0.4 % concentrations. Heating the virus-infected samples or treating them with ultraviolet light — previously recommended by some virologists and agricultural agencies — took a longer time.
For example, the virus was killed after 30 minutes at 56°C and after one day at 28 °C. The virus remained viable even after an hour of exposure to ultraviolet light exposure.
Dr. Muhammad Akbar Shahid, the leading author of the study, and now a microbiologist at the Bahauddin Zakariya University, Pakistan, said that frequent washing of hands and face with soap can destroy the virus and prevent the spread of any respiratory virus including H5N1 (bird flu virus) and novel coronavirus; however, use of other measures including wearing of face masks, observing a safe distance from the infected carriers (either birds or humans) still need to be practiced.
How simple soaps and detergents can be so lethal against the bird flu and SARS-CoV-2 viruses?
According to experts, washing hands, for at least twenty seconds, is the most-effective method to save us from novel coronavirus.
So, the question arises as to how simple soaps and detergents can be so lethal against the bird flu and SARS-CoV-2 viruses?
The short answer is that a virus is a nanoparticle which is an assembly of ribonucleic acid (RNA), proteins and lipids. In this assembly, the weakest link is the lipid or fatty bilayer. Soap just dissolves this fat layer, and the virus is broken apart — and we call it as if the virus is dead which is actually inactive as viruses are not really ‘alive’.
To understand how soaps and detergents kill viruses including bird flu and SARS-CoV-2, we have to understand a little bit of science. The molecules of soaps are known as “amphiphiles” and the amphiphiles are made of two words: “amphis” (which means “both”) and “philia” (kind of “love”).
The molecule of soap, and possibly detergent, has two ends with very unique properties. One end is called polar (water-loving or hydrophilic) which is fat-resistant, while the other end is called nonpolar which is fat-loving (lipophilic) and water-resistant.
One end of the amphiphile molecule is attracted to the water but is repelled by proteins and fats. The other end is attracted to fats and proteins but is repelled by water. If we pour fat-containing olive oil into water, the fats don’t mix with water. But if we add some soap to oil-and-water, the soap molecules form a ring around the oil. This ring is called a ‘micelle’.
In micelle, soap is attracted to the oil due to its nonpolar side. Then it starts to tear it up and pull it into the water due to its polar or water-loving side.
Watch this video to understand how it works:
How can we relate this knowledge with the destruction of the coronavirus or bird flu virus by soaps and detergents? We can compare the coronavirus SARS-CoV-2, bird flu virus or any other enveloped virus, to the drops of olive oil in water. The virus contains genetic information, encoded by either RNA or DNA (some viruses), encased in a coating of fat and protein. To understand the nature of this tiny virus, we can call it nano-size grease balls.
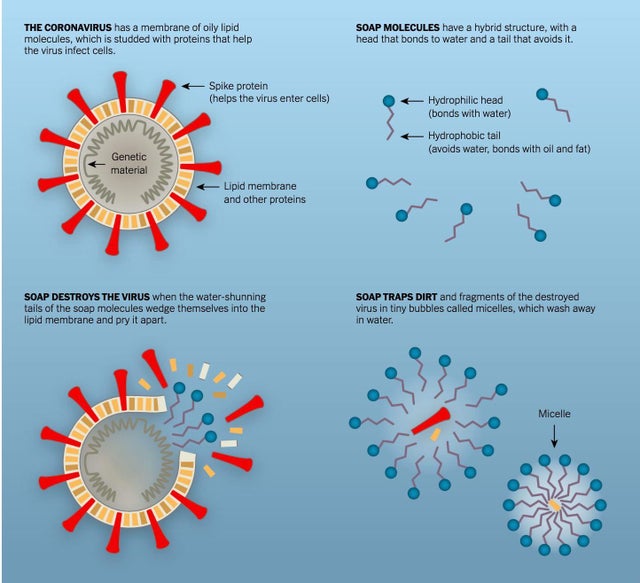
The way soap destroys the grease ball, similarly, soap meets a COVID-19-causing virus. The soap molecules enter the virus’ lipid bilayer (double layer) envelope or coating. The nonpolar side of a soap molecule attacks the virus by inserting it into its fat-and-protein layer. Because the chemical bonds which hold this particular virus together are fairly weak, the molecular invasion is sufficient to break the virus’s coating. Once the virus’s coating is broken, the virus gets pulled apart. When the virus is broken apart, it becomes water-soluble. A water-soluble virus is a decaying virus.
That’s how soap can destroy the SARS-CoV-2 virus. But — it takes time, approximately 20 seconds.
Why soaps and detergents take so much time?
Here the question arises: Why so much time is taken by soaps and detergents to kill the virus? If we look at our fingertips, we see small folds and wrinkles. Our fingertips aren’t flat. These folds and wrinkles may provide hiding places for the viruses.
To ensure soap interacts with and destroys the virus particles, we should not apply soap and rinse quickly, rather we should give at least 20 seconds for the soap to interact with the virus so that it has been distorted into harmless remains and then washed with water into the drain.
Importance of handwashing with soaps and detergents
Our hands are attraction points for various types of viruses and bacteria. Even if soap cannot kill every single type of invisible germ, hiding within the folds on our fingertips — like soap kills the bird flu and coronavirus — the habit of properly washing our hands and faces with soap will at least keep those viruses and bacteria away from our us and cause serious diseases.
We touch our faces (eyes, mouth, nostrils) with our hands approximately 23 times per hour. That’s the way viruses — like the novel coronavirus SARS-CoV-2 — or any other deadly virus or bacteria enter our bodies and cause a dreadful disease. Therefore, it is very important to keep our hands clean by using soap.
Further reading: Shahid, M.A., Abubakar, M., Hameed, S. et al. Avian influenza virus (H5N1); effects of physico-chemical factors on its survival. Virol J 6, 38 (2009). https://doi.org/10.1186/1743-422X-6-38
Leave a Reply